The development of the eye during childhood is controlled by visual input such that the power of the eye matches the size of the ocular globe to focus light on the retina. While this process is well known, the mechanisms behind this control remain elusive. The exact aspects of the visual environment that signal eye growth are unknown, although it has been shown that local retinal circuits detect the visual input and send a growth signal to the sclera to alter the size of the eye. To further our understanding of visually-driven eye growth and myopia, our experiments address these questions:
What are the retinal mechanisms that underlie refractive development and myopia?
In the last 30 years there has been a rapid increase in the prevalence of myopia in many countries around the world. Rates of individuals affected by myopia are as high as 86% in some Asian countries. Since refractive errors reduce visual acuity, it has been assumed that cone pathways that underlie high visual acuity are necessary for normal eye growth. Our research and that of others is challenging this assumption. Our lab is using a new animal model in the field of experimental myopia: the mouse (Figure 1).
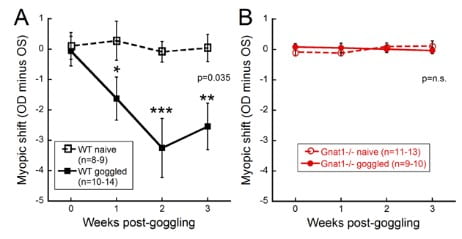
Like other animal models, the mouse responds to form deprivation or lens defocus by developing myopia. However, the mouse model of myopia offers the unique ability to isolate specific genes, in addition to manipulating the experimental environment. We are examining the contribution of specific retinal cell types and pathways such as photoreceptors (rods, cones and intrinsically photosensitive retinal ganglion cells), and ON and OFF pathways. Using mutant mouse models, we have shown that rod pathways are critical to responding to form deprivation (Figure 2; Park et al., IOVS 2014) and that ON pathways influence refractive development (Pardue et al., IOVS 2008; Chakraborty et al., EER 2015) more than OFF pathways (Chakraborty et al., MolVision 2014).
Current and future experiments are examining the potential signaling mechanisms that underlie these pathways, like dopamine.
Are disrupted circadian rhythms contributing to myopia?
In collaboration with Dr. Richard Stone at University of Pennsylvania and Dr. Michael Iuvone at Emory University, we have been using mice with specific mutations in the clock genes that are essential for circadian rhythms and determining their influence on normal refractive development and form deprivation. These experiments are ongoing and continue to increase our understanding of the mechanisms underlying refractive development.
Can visual stimuli be rehabilitative for refractive eye growth?
In order to combat the dramatic increase in myopia, our lab is examining ways to modulate refractive development through the use of ambient light levels, image contrast and circadian rhythms. These studies suggest that different visual environments can influence whether an eye develops myopia. Future experiments are examining these aspects and the potential underlying mechanisms to develop therapeutic strategies for myopia.